Abstract
BACKGROUND: Hyperuricemia is a major cause of tumor lysis syndrome (TLS) related acute kidney injury. Certain hematologic malignancies (acute leukemia, high-grade lymphoma) pose a high-risk for spontaneous and chemotherapy-induced TLS. Allopurinol, a xanthine oxidase inhibitor, can inhibit formation of new uric acid (UA), but it does not eliminate the already formed UA. Alternatively, urate oxidases like rasburicase (ELITEK®, Sanofi Genzyme) can markedly and rapidly decrease UA levels through oxidation of UA to allantoin and hydrogen peroxide. In a phase III trial, rasburicase therapy more rapidly controlled UA than allopurinol in adults with hyperuricemia or at high-risk for TLS. However, rasburicase is expensive, especially when used as per the manufacturer's recommended dosage of 0.2mg/kg/day for up to 5 days. Numerous reports have shown that lower, and even single, doses are effective in lowering UA levels.
OBJECTIVES: To determine the efficacy and safety of two single low doses of rasburicase (1.5mg vs. 3mg) in adult patients with acute leukemia and elevated plasma uric acid ≥ 7.5mg/dL.
METHODS: Patients ≥ 18 years treated inpatient for acute leukemia with UA ≥ 7.5 mg/dL and at risk for TLS were randomized to one of two treatment arms (ClinicalTrials.gov Identifier: NCT01564277). Patients received either rasburicase 1.5mg (Arm A) or rasburicase 3mg (Arm B) intravenously over 30 minutes on day 1 in an open-label and unblinded fashion. All patients received allopurinol 300mg daily on days 1-6 (dose reduced for reduced calculated creatinine clearance). Plasma samples for UA evaluation were collected at baseline, and at approximately 4, 24, 48, 72, 96, 120, 144 hours after the Day 1 dose of rasburicase. If UA > 7.5 mg/dL at 24 or 48 hours, then additional dose of rasburicase could be administered (1.5mg for Arm A, 3mg for Arm B). Primary endpoint was the response rate of obtaining UA ≤ 7.5mg/dL within 24 hrs of rasburicase treatment. Secondary endpoints included rate of patients that maintained UA ≤ 7.5 mg/dl on days 2-6, rate of patients who required additional rasburicase doses, rate of patients experiencing significant worsening of renal function, as well as safety of low single-dose rasburicase. Patients were followed for adverse events (AE; as defined in CTCAE version 4) for up to 30 days after last dose of rasburicase or resolution of drug-related AE.
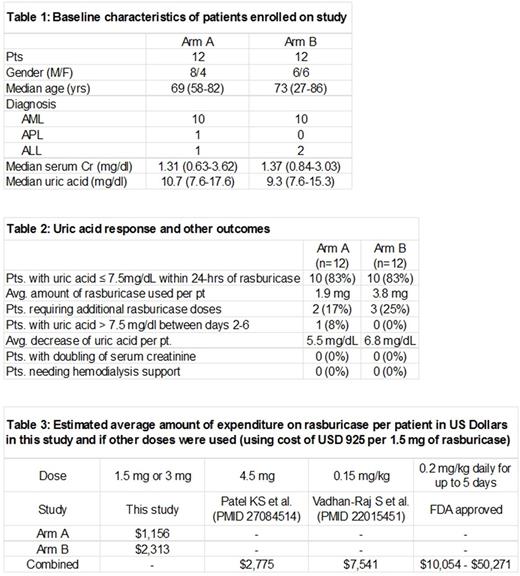
RESULTS: 24 patients were enrolled on this phase 2 study (12 on each arm; Table 1). 83% of patients in both arms achieved UA response by 24 hours (Table 2). Two pts in Arm A (baseline UA: 14.2 and 17.6 mg/dL) and three pts in Arm B (baseline UA: 15.3, 10.2, 11.9 mg/dL) were given one additional rasburicase dose. Only one pt (from Arm A) had UA > 7.5 mg/dL between days 2-6. None of the patients had doubling of their baseline serum creatinine or required hemodialysis support. Average decrease in UA was lower in Arm A as compared to Arm B (5.5 vs. 6.8 mg/dL). AE data from 21 pts showed that both low doses were overall well tolerated and most toxicities were unrelated to rasburicase. No patient deaths were attributed to rasburicase. An ad-hoc analysis showed significant rasburicase specific cost benefits using either of the low doses as compared to FDA approved dosage and other studied regimens (Table 3).
CONCLUSIONS: Single low doses of rasburicase (1.5mg and 3mg) plus allopurinol are well tolerated and appear to be equally efficacious in decreasing UA levels in adult patients with leukemia at high risk for TLS. If baseline UA is elevated (>12 mg/dL), a 3mg or higher dose of rasburicase might be preferable. Preliminary cost analysis profiling favors the use of the low dose rasburicase.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal